Only ENVARSUS XR is created with proprietary MeltDose® technology to deliver smooth, extended-release tacrolimus absorption over 24 hours.2,3
A smaller particle size allows for optimized dissolution, absorption, and oral bioavailability as compared with other conventional drugs.3
ENVARSUS XR IS ABSORBED THROUGHOUT THE ENTIRE GI TRACT3,5
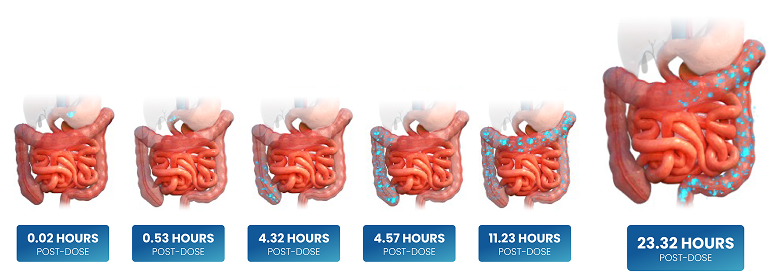
Illustrations of scintigraphy data showing that dissolution of ENVARSUS XR takes place throughout the gut5
These images are illustrations of scintigraphy data showing that dissolution of ENVARSUS XR takes place throughout the gut.5
Scintigraphic images were acquired at approximately 10- to 15-minute intervals until 8 hours post dose, then at 30-minute intervals until 16 hours post dose, and then at 24, 36, 48, and 72 hours post dose.5 CYP3A5 activity is greatest in the proximal gastrointestinal region and decreases downstream throughout the bowel.6 Therefore, the delayed absorption throughout the gastrointestinal tract results in decreased presystemic metabolism.5,6
GI=gastrointestinal; PK=pharmacokinetic.
See how ENVARSUS XR works
Watch the ENVARSUS XR mechanism of Delivery video
Explore the mechanism of delivery for ENVARSUS XR in the prophylaxis of organ rejection in de novo kidney patients in combination with other immunosuppressants and the prophylaxis of organ rejection in kidney transplant patients converted from tacrolimus immediate-release formulations in combination with other immunosuppressants.
[VOICEOVER]
(On-screen text reads, “IR-TACROLIMUS. FDA approval in 1994. ONE OF THE MOST WIDELY USED IMMUNOSUPPRESSANT DRUGS IN KIDNEY TRANSPLANTATION”)
Initially approved by the FDA in 1994, tacrolimus is one of the most widely used immunosuppression drugs in kidney transplantation.
(On-screen text reads, “HIGHLY EFFECTIVE IN PREVENTING GRAFT REJECTION”)
Although immediate-release tacrolimus has been proven highly effective in preventing graft rejection, it is not without limitations.
(On-screen text reads, “LIMITATION”)
Immediate-release tacrolimus has low bioavailability,
(On-screen text reads, “Low Bioavailability. Less than 20% of an orally administered dose. Absorption of tacrolimus from the gastrointestinal tract after oral administration is incomplete and variable. PROGRAF [prescribing information]. Northbrook, IL: Astellas Pharma US, Inc.; 8/2023)
often reported to be less than 20% of an orally administered dose.
The low bioavailability of tacrolimus is largely due to the high rate of presystemic metabolism. Presystemic metabolism is primarily the result of 2 systems, which are active in the transport and metabolism of drugs from the GI tract.
(On-screen text reads, “P-glycoprotein and cytochrome P450 systems. ENVARSUS XR [prescribing information]. Cary, NC: Veloxis Pharmaceuticals, Inc.; 4/2024”)
This first system is the P-glycoprotein system, which is responsible for transporting the drug out of cells and back into the lumen of the intestine.
(On-screen text reads: “LUMEN, PGP, ENTEROCYTE”)
The other active system is the cytochrome P450 isoenzyme system, which is a group of enzymes that converts tacrolimus into predominately inactive metabolites. P450 enzymes are present both on the intestinal luminal cells as well as in the liver, affecting both presystemic tacrolimus and tacrolimus absorbed into the bloodstream.
(On-screen text reads: “CYP450” “Effecting both presystemic tacrolimus and tacrolimus absorbed into the blood stream” “UNCHANGED DRUG” “INACTIVE METABOLITE” “BLOOD STREAM”)
Importantly, only tacrolimus in the bloodstream, in its unchanged form, is able to suppress T cells and prevent rejection.
(On-screen text reads, “Only tacrolimus in the bloodstream in its unchanged form is able to suppress T cells and prevent rejection”)
In reality, these 2 systems function simultaneously to transport and metabolize tacrolimus so effectively that only about 20% of the drug is absorbed into the bloodstream.
(On-screen text reads, “Only about 20% of the unchanged drug is absorbed into the bloodstream”)
This limited oral bioavailability of tacrolimus creates clinical challenges for the transplant clinician.
Introducing ENVARSUS XR controlled release with MeltDose® technology for stable delivery.
(On-screen text reads, “Introducing ENVARSUS XR® and MeltDose® technology”)
Please see Boxed Warning regarding malignancies and serious infections, as well as additional full Important Safety Information, at the end of this video.
(On-screen text reads,
“INDICATIONS AND USAGE
ENVARSUS XR is indicated for the prophylaxis of organ rejection in de novo kidney transplant patients in combination with other immunosuppressants.
ENVARSUS XR is also indicated for the prophylaxis of organ rejection in kidney transplant patients converted from tacrolimus immediate-release formulations in combination with other immunosuppressants.
IMPORTANT SAFETY INFORMATION
BOXED WARNING:
WARNING: MALIGNANCIES AND SERIOUS INFECTIONS
Increased risk for developing serious infections and malignancies with ENVARSUS XR or other immunosuppressants that may lead to hospitalization or death
CONTRAINDICATIONS
ENVARSUS XR is contraindicated in patients with known hypersensitivity to tacrolimus or to any of the ingredients in ENVARSUS XR.
See full Important Safety Information, including Boxed Warning, at the end of this video.”)
(On-screen text reads, “MeltDose® technology” “A specially designed carrier matrix”)
ENVARSUS XR uses MeltDose® technology to apply single-molecule tacrolimus to a specially designed carrier matrix. This allows it to be held in a solid suspension and released in a delayed fashion over the length of the GI tract.
(On-screen text reads, “The differences in the THREE TACROLIMUS FORMULATIONS Time to peak” “PROGRAF®” “ASTAGRAF XL®” “ENVARSUS XR®” “Tremblay S, Nigro V, Weinberg J, Woodle ES, Alloway RR. A steady-state head-to-head pharmacokinetic comparison of all FK-506 (tacrolimus) formulations (ASTCOFF): an open-label, prospective, randomized, two-arm, three-period crossover study. Am J Transplant. 2017;17(2):432-442. Normalized to mean whole blood concentration of tacrolimus based on conversion factors of 1 (PROGRAF): 1.08 (ASTAGRAF XL): 0.7 (ENVARSUS XR).”)
To understand the impact of formulation on 24-hour pharmacokinetics, a study was done to assess the 3 tacrolimus formulations. It entailed frequent assessment of tacrolimus levels over a 24-hour period.
As demonstrated in this study, immediate-release tacrolimus achieves maximum concentrations at 1.5 hours and declines rapidly until the evening dose is administered, 12 hours later. There is long-standing evidence with immediate-release tacrolimus that diurnal variation and bioavailability exists. Thus the nighttime dose is typically less bioavailable and contributes less to the overall, 24-hour exposure.
(On-screen text reads, [HEADER]“PROGRAF”
[LINE GRAPH: Y-AXIS]“Mean Whole Blood Concentration (ng/mL) 5, 10, 15, 20, 25” [CHART LEGEND]“PROGRAF” [LINE GRAPH: X-AXIS]“Time (hr) 0, 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, 24” [FOOTER] “Tremblay S, Nigro V, Weinberg J, Woodle ES, Alloway RR. A steady-state head-to-head pharmacokinetic comparison of all FK-506 (tacrolimus) formulations (ASTCOFF): an open-label, prospective, randomized, two-arm, three-period crossover study. Am J Transplant. 2017;17(2):432-442. Normalized to mean whole blood concentration of tacrolimus based on conversion factors of 1 (PROGRAF): 1.08 (ASTAGRAF XL): 0.7 (ENVARSUS XR).”)
In an attempt to delay and lower the peak, while providing similar overall exposure, sustained release dosage forms were created. ASTAGRAF results in slightly lower and slightly delayed peaks, though these were not found to be significantly different than immediate-release tacrolimus.
(On-screen text reads, [HEADER]“ASTAGRAF XL”
[LINE GRAPH: Y-AXIS]“Mean Whole Blood Concentration (ng/mL) 5, 10, 15, 20, 25” [CHART LEGEND]“ASTAGRAF XL” [LINE GRAPH: X-AXIS]“Time (hr) 0, 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, 24” [FOOTER] “Tremblay S, Nigro V, Weinberg J, Woodle ES, Alloway RR. A steady-state head-to-head pharmacokinetic comparison of all FK-506 (tacrolimus) formulations (ASTCOFF): an open-label, prospective, randomized, two-arm, three-period crossover study. Am J Transplant. 2017;17(2):432-442. Normalized to mean whole blood concentration of tacrolimus based on conversion factors of 1 (PROGRAF): 1.08 (ASTAGRAF XL): 0.7 (ENVARSUS XR).”)
ENVARSUS XR was designed to deliver the drug throughout the GI tract, creating a truly delayed drug delivery to the patient. Given the controlled release of tacrolimus from ENVARSUS XR tablets, ENVARSUS XR results in significantly lower maximum concentrations, a longer time to peak, and less fluctuation in level, while still delivering a similar overall exposure over 24 hours.
(On-screen text reads, [HEADER]“ENVARSUS XR Impact of MeltDose®: Target exposure with a significantly lower peak” [LINE GRAPH: Y-AXIS]“Mean Whole Blood Concentration (ng/mL) 5, 10, 15, 20, 25” [CHART LEGEND] “PROGRAF” “ASTAGRAF XL” “ENVARSUS XR” [LINE GRAPH: X-AXIS]“Time (hr) 0, 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, 24” [FOOTER] “Tremblay S, Nigro V, Weinberg J, Woodle ES, Alloway RR. A steady-state head-to-head pharmacokinetic comparison of all FK-506 (tacrolimus) formulations (ASTCOFF): an open-label, prospective, randomized, two-arm, three-period crossover study. Am J Transplant. 2017;17(2):432-442. Normalized to mean whole blood concentration of tacrolimus based on conversion factors of 1 (PROGRAF): 1.08 (ASTAGRAF XL): 0.7 (ENVARSUS XR). Clinical benefit of the differences in ENVARSUS XR PK has not been established”)
In subsequent Phase 3 clinical studies, results demonstrated that once-daily ENVARSUS XR was noninferior to twice-daily immediate-release Prograf® in treatment failure in de novo renal transplant patients. The clinical benefit of the differences in ENVARSUS XR PK has not been established.
(On-screen text reads, [HEADER]“Patients remaining free from treatment failure TIME FREE FROM TREATMENT FAILURE VS PROGRAF” [LINE GRAPH: Y-AXIS]“% Patients 50, 60, 70, 80, 90, 100” [CHART LEGEND] “ENVARSUS XR (n=268)” “PROGRAF (n=275)” [PLOT POINT 1]“HR 0.91 (95% CI, 0.62-1.34)” [PLOT POINT 2]“HR 0.83 (95% CI, 0.59-1.16)” [LINE GRAPH: X-AXIS]“Days after randomization 0, 30, 60, 90, 120, 180, 240, 300, 360, 420, 480, 540, 600, 660, 720” [FOOTER] “The clinical benefit of the differences in ENVARSUS XR PK has not been established” “Rostaing L, Bunnapradist S, Grinyó JM, et al. Novel once-daily extended-release tacrolimus versus twice-daily tacrolimus in de novo kidney transplant recipients: two-year results of phase 3, double-blind, randomized trial. Am J Kidney Dis. 2016;67(4):648-659.”)
ENVARSUS XR. Consider the impact of this technology for your transplant patients.
INDICATIONS AND USAGE
ENVARSUS XR is indicated for the prophylaxis of organ rejection in de novo kidney transplant patients in combination with other immunosuppressants.
ENVARSUS XR is also indicated for the prophylaxis of organ rejection in kidney transplant patients converted from tacrolimus immediate-release formulations in combination with other immunosuppressants.
IMPORTANT SAFETY INFORMATION
BOXED WARNING:
MALIGNANCIES AND SERIOUS INFECTIONS
Increased risk for developing serious infections and malignancies with ENVARSUS XR or other immunosuppressants that may lead to hospitalization or death
CONTRAINDICATIONS
ENVARSUS XR is contraindicated in patients with known hypersensitivity to tacrolimus or to any of the ingredients in ENVARSUS XR.
WARNINGS AND PRECAUTIONS
Lymphoma and Other Malignancies: Immunosuppressants, including ENVARSUS XR, increase the risk of developing lymphomas and other malignancies, particularly of the skin. Post-transplant lymphoproliferative disorder (PTLD), associated with Epstein-Barr Virus (EBV), has been reported in immunosuppressed organ transplant patients.
Serious Infections: Immunosuppressants, including ENVARSUS XR, increase the risk of developing bacterial, viral, fungal, and protozoal infections, including opportunistic infections. These infections may lead to serious, including fatal, outcomes.
Not Interchangeable with Other Tacrolimus Products - Medication Errors: Medication errors, including substitution and dispensing errors, between tacrolimus capsules and tacrolimus extended-release capsules were reported outside the U.S. in some cases leading to adverse reactions. ENVARSUS XR is not interchangeable or substitutable with tacrolimus extended-release capsules, tacrolimus capsules or tacrolimus for oral suspension.
New Onset Diabetes after Transplant: ENVARSUS XR caused new onset diabetes after transplant (NODAT) in kidney transplant patients, which may be reversible in some patients. African-American and Hispanic kidney transplant patients are at an increased risk.
Nephrotoxicity due to ENVARSUS XR and Drug Interactions: ENVARSUS XR, like other calcineurin-inhibitors, can cause acute or chronic nephrotoxicity. In patients with elevated serum creatinine and tacrolimus whole blood trough concentrations greater than the recommended range, consider dosage reduction or temporary interruption of tacrolimus administration. The risk for nephrotoxicity may increase when ENVARSUS XR is concomitantly administered with CYP3A inhibitors (by increasing tacrolimus whole blood concentrations) or drugs associated with nephrotoxicity. When tacrolimus is used concurrently with CYP3A inhibitors or other known nephrotoxic drugs, monitor renal function and tacrolimus blood concentrations, and adjust dose of both tacrolimus and/or concomitant medications during concurrent use.
Neurotoxicity: ENVARSUS XR may cause a spectrum of neurotoxicities. The most severe neurotoxicities include posterior reversible encephalopathy syndrome (PRES), delirium, seizure, and coma; others include tremors, paresthesias, headache, mental status changes, and changes in motor and sensory functions.
Hyperkalemia: Mild to severe hyperkalemia, which may require treatment, has been reported with tacrolimus including ENVARSUS XR. Concomitant use of agents associated with hyperkalemia may increase the risk for hyperkalemia.
Hypertension: Hypertension is a common adverse reaction of ENVARSUS XR therapy and may require antihypertensive therapy.
[TEXT ON-SCREEN]
Risk of Rejection with Strong CYP3A Inducers and Risk of Serious Adverse Reactions with Strong CYP3A Inhibitors: The concomitant use of strong CYP3A inducers may increase the metabolism of tacrolimus, leading to lower whole blood trough concentrations and greater risk of rejection. In contrast, the concomitant use of strong CYP3A inhibitors may decrease the metabolism of tacrolimus, leading to higher whole blood trough concentrations and greater risk of serious adverse reactions. Therefore, adjust ENVARSUS XR dose and monitor tacrolimus whole blood trough concentrations when co-administering ENVARSUS XR with strong CYP3A inhibitors or strong CYP3A inducers. A rapid, sharp rise in tacrolimus levels has been reported after co-administration with strong CYP3A4 inhibitors despite an initial reduction of tacrolimus dose. Early and frequent monitoring of tacrolimus whole blood trough levels is recommended.
QT Prolongation: ENVARSUS XR may prolong the QT/QTc interval and cause Torsade de pointes. Avoid ENVARSUS XR in patients with congenital long QT syndrome. Consider obtaining electrocardiograms and monitoring electrolytes periodically during treatment in patients with congestive heart failure, bradyarrhythmias, those taking certain antiarrhythmic medications or other products that lead to QT prolongation, and those with electrolyte disturbances. When co-administering ENVARSUS XR with other substrates and/or inhibitors of CYP3A, especially those that also have the potential to prolong the QT interval, a reduction in ENVARSUS XR dosage, monitoring of tacrolimus whole blood concentrations, and monitoring for QT prolongation is recommended.
Immunizations: Whenever possible, administer the complete complement of vaccines before transplantation and treatment with ENVARSUS XR. Avoid the use of live attenuated vaccines during treatment with ENVARSUS XR. Inactivated vaccines noted to be safe for administration after transplantation may not be sufficiently immunogenic during treatment with ENVARSUS XR.
Pure Red Cell Aplasia: Cases of pure red cell aplasia (PRCA) have been reported in patients treated with tacrolimus. If PRCA is diagnosed, consider discontinuation of ENVARSUS XR.
Cannabidiol Drug Interactions: When cannabidiol and ENVARSUS XR are co-administered, closely monitor for an increase in tacrolimus blood levels and for adverse reactions suggestive of tacrolimus toxicity. A dose reduction of ENVARSUS XR should be considered as needed when ENVARSUS XR is co-administered with cannabidiol.
Thrombotic Microangiopathy (TMA) Including Hemolytic Uremic Syndrome and Thrombotic Thrombocytopenic Purpura: Cases of thrombotic microangiopathy (TMA), including hemolytic uremic syndrome (HUS) and thrombotic thrombocytopenic purpura (TTP), have been reported in patients treated with ENVARSUS XR. Transplant patients may have other risk factors which contribute to the risk of TMA. In patients with signs and symptoms of TMA, consider ENVARSUS XR as a risk factor. Concurrent use of ENVARSUS XR and mammalian target of rapamycin (mTOR) inhibitors may contribute to the risk of TMA.
ADVERSE REACTIONS
De Novo kidney transplant patients: Most common adverse reactions (incidence ≥15%) reported with ENVARSUS XR are diarrhea, anemia, urinary tract infection, hypertension, tremor, constipation, diabetes mellitus, peripheral edema, hyperkalemia and headache.
Conversion of kidney transplant patients from immediate-release tacrolimus: Most common adverse reactions (incidence ≥10%) reported with ENVARSUS XR include: diarrhea and blood creatinine increased.
USE IN SPECIFIC POPULATIONS
Pregnancy: Based on postmarketing surveillance, registry and animal data may cause fetal harm. Advise pregnant women of the potential risk to the fetus.
Nursing Mothers: Tacrolimus is present in human milk. Discontinue drug or nursing, taking into account the importance of drug to the mother.
Females and Males of Reproductive Potential: Advise female and male patients of reproductive potential to speak with their healthcare provider on family planning options including appropriate contraception prior to starting treatment with ENVARSUS XR. Based on animal studies, ENVARSUS XR may affect fertility in males and females.
Pediatric Use: The safety and efficacy of ENVARSUS XR in pediatric patients have not been established.
Geriatric Use: Clinical studies of ENVARSUS XR did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients.
Renal Impairment: Frequent monitoring of renal function is recommended. Lower doses may be required.
Hepatic Impairment: Frequent monitoring of tacrolimus trough concentrations is recommended. With greater tacrolimus whole blood trough concentrations in patients with severe hepatic impairment, there is a greater risk of adverse reactions and dosage reduction is recommended.
Race: African-American patients may require higher doses to attain comparable trough concentrations compared to Caucasian patients. African-American and Hispanic kidney transplant patients are at an increased risk for new onset diabetes after transplant. Monitor blood glucose concentrations and treat appropriately.
To report SUSPECTED ADVERSE REACTIONS, contact Veloxis Pharmaceuticals, Inc. at 1-844-VELOXIS (835-6947) or FDA at 1-800-FDA-1088 or visit www.fda.gov/medwatch.
Please see full Prescribing Information, including Boxed Warning.
A smooth and consistent PK profile you—
and your patients—can count on
50% greater bioavailability vs PROGRAF® or ASTAGRAF®2
ENVARSUS XR has been shown to achieve target trough levels with a 20% lower total daily dose vs immediate-release tacrolimus7
In addition to a lower total daily dose, ENVARSUS XR reaches target exposure with a significantly lower peak vs PROGRAF or ASTAGRAF2*
Study Design: Open-label, randomized, 2-sequence, 3-period crossover trial of adult stable kidney transplant patients (N=32). The primary objective of the study was to evaluate the PK profile of ENVARSUS XR vs PROGRAF and ASTAGRAF XL.
Clinical benefit of the differences in the PK profile of ENVARSUS XR has not been established.
*Normalized to mean whole blood concentrations of tacrolimus based on conversion factors of 1 (PROGRAF), 1.08 (ASTAGRAF XL), 0.7 (ENVARSUS XR).


Read the Consistency Case Study
This case study highlights a patient and her care team’s experiences maintaining tacrolimus levels at the high end of the therapeutic range.
Download Case StudyConsistent PK, even in rapid metabolizers
ENVARSUS XR provides consistent pharmacokinetics across all patient types—even hard-to-treat patients, such as rapid metabolizers.
Who Are Rapid Metabolizers?
In the ASERTAA Phase 3b pharmacogenetic study of African American kidney transplant recipients, rapid metabolizers* taking ENVARSUS XR experienced 30% lower peak concentrations compared with IR-Tac.1
Study Design: Phase 3b prospective, randomized, open-label, 2-sequence, 3-period, crossover pharmacogenetic study to compare the steady state PK of IR-Tac twice daily to ENVARSUS XR once daily in adult stable African American kidney transplant patients (N=46). Patients were randomized to receive either IR-Tac for 7 days and then switched to ENVARSUS XR for 14 days or ENVARSUS XR for 7 days and switched to IR-Tac for 14 days. Patients continued concomitant immunosuppression per standard of care. Patients were genotyped and PK assessments were completed on study days 7, 14, and 21.1
IR-Tac=immediate-release tacrolimus.
*Definitions of "rapid metabolizer" may differ from study to study.
Clinical benefit of the differences in the PK profile of ENVARSUS XR has not been established.


Review Rapid–Metabolizer Data
Review the results of ASERTAA, a Randomized Prospective Crossover Pharmacogenetic Study of Immediate-Release Tacrolimus Versus Extended-Release Tacrolimus in African American Kidney Transplant Recipients
Read the StudyReferences: 1. Trofe-Clark J, Brennan DC, West-Thielke P, et al. Results of ASERTAA, a randomized prospective crossover pharmacogenetic study of immediate-release versus extended-release tacrolimus in African American kidney transplant recipients. Am J Kidney Dis. 2018;71(3):315-326. 2. Tremblay S, Nigro V, Weinberg J, Woodle ES, Alloway RR. A steady-state head-to-head pharmacokinetic comparison of all FK-506 (tacrolimus) formulations (ASTCOFF): an open-label, prospective, randomized, two-arm, three-period crossover study. Am J Transplant. 2017;17(2):432-442. 3. Grinyó JM, Petruzzelli S. Once-daily LCP-tacro MeltDose tacrolimus for the prophylaxis of organ rejection in kidney and liver transplantations. Expert Rev Clin Immunol. 2014;10(12):1567-1579. 4. Data on file. Veloxis Pharmaceuticals, Inc.; 2018. 5. Nigro V, Glicklich A, Weinberg J. Improved bioavailability of MELTDOSE once-daily formulation of tacrolimus (LCP-Tacro) with controlled agglomeration allows for consistent absorption over 24 hrs: a scintigraphic and pharmacokinetic evaluation [abstract]. Am J Transplant. 2013;13(suppl 5):335.
6. Thörn M, Finnström N, Lundgren S, Rane A, Lööf L. Cytochromes P450 and MDR1 mRNA expression along the human gastrointestinal tract. Br J Clin Pharmacol. 2005;60(1):54-60. 7. ENVARSUS XR [package insert]. Cary, NC: Veloxis Pharmaceuticals, Inc.; 4/2024. 8. Staatz C, Goodman LK, Tett SE. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin Inhibitors: part I. Clin Pharmacokinet. 2010;49(3):141-175. 9. Kuehl P, Zhang J, Lin Y, et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet. 2001;27(4):383-391.


