Simplified, once-daily dosing
ENVARSUS XR is available in a variety of tablet strengths and package sizes to meet the needs of patients and customers.2
0.75-mg tablet, 1-mg tablet, and 4-mg tablet of ENVARSUS XR.*
*Tablets not shown at actual size.
Convert with confidence
Determine the correct ENVARSUS XR dose for your patient using our convenient dose conversion calculator.
A dosing regimen designed to support adherence
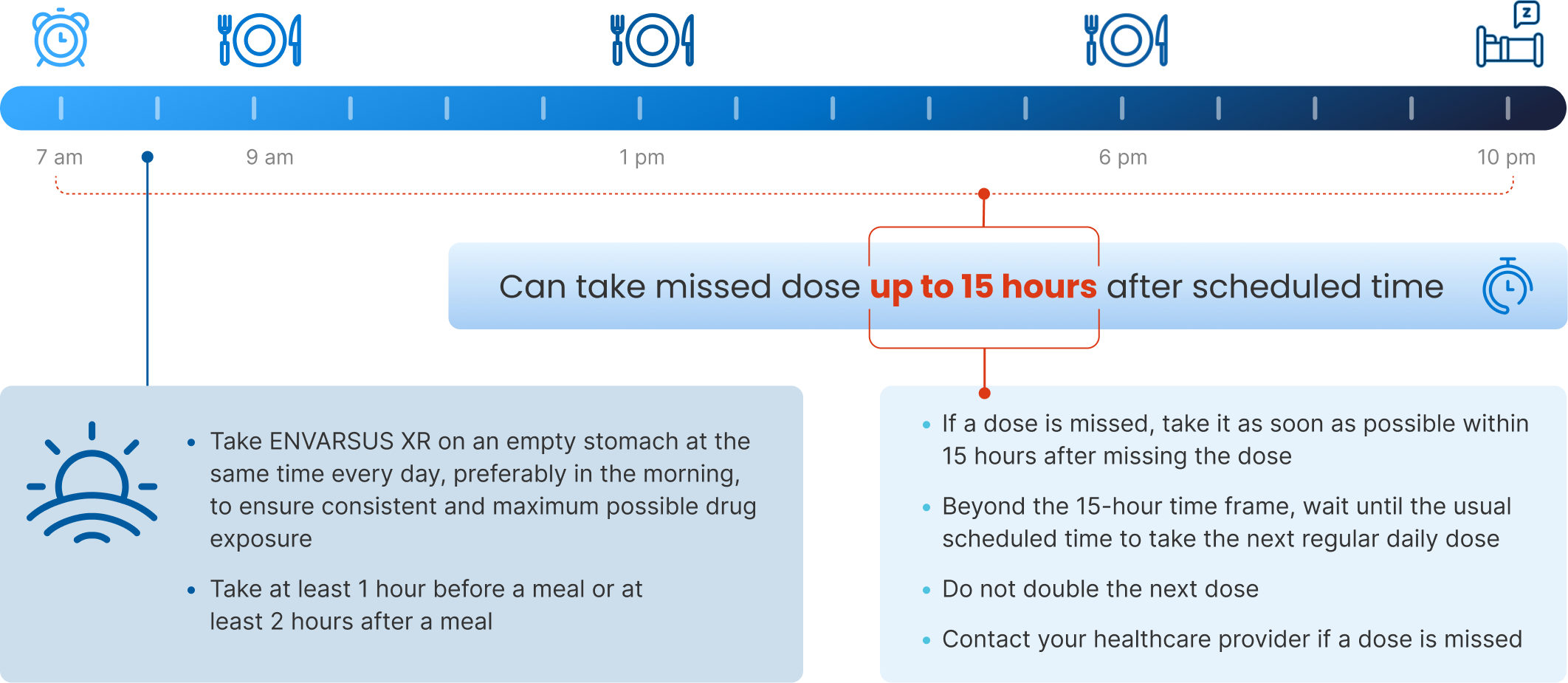
Important administration instructions:
- There is no generic equivalent for ENVARSUS XR3
- ENVARSUS XR (tacrolimus extended-release tablets) is not interchangeable or substitutable with tacrolimus extended-release capsules, tacrolimus capsules, and tacrolimus for oral suspension. Under- or overexposure to tacrolimus may result in graft rejection or other serious adverse reactions
- ENVARSUS XR should not be used without the supervision of a physician with experience in immunosuppressive therapy
- ENVARSUS XR should be taken on an empty stomach consistently at the same time of the day, preferably in the morning to ensure consistent and maximum possible drug exposure, at least 1 hour before a meal or at least 2 hours after a meal
- Advise patients to swallow ENVARSUS XR tablets whole with fluid (preferably water); patients must not chew, divide, crush, or dissolve the tablets
- If a dose is missed, instruct the patient to take it as soon as possible within 15 hours after missing the dose. Beyond the 15-hour time frame, instruct the patient to wait until the usual scheduled time to take the next regular daily dose. Instruct the patient not to double the next dose
- Patients should avoid eating grapefruit or drinking grapefruit juice or alcoholic beverages while taking ENVARSUS XR
Dosing for de novo kidney transplant patients
- For de novo patients (with antibody induction), the recommended starting dose is 0.14 mg/kg/day2
- Dosing adjustments to consider when administered concomitantly with CYP3A inducers or inhibitors
- Patients with severe hepatic impairment may require a lower starting dose2
- African American patients may need to be titrated to higher ENVARSUS XR dosages to attain comparable trough concentrations2
- Should be taken once daily on an empty stomach, preferably in the morning, at least 1 hour before or 2 hours after a meal
- Recommended target whole blood trough concentration range
- Month 1: 6 to 11 ng/mL
- After Month 1: 4 to 11 ng/mL

Switching to ENVARSUS XR is a straightforward process
ENVARSUS XR is not interchangeable with any other tacrolimus brand or generic product.2 However, even for patients already on a different medication regimen (like IR-Tac), switching to ENVARSUS XR is a simple and straightforward process.2
Administer 80% of the preconversion daily dose2
hidden text
- Start ENVARSUS XR at 80% of the total daily dose of immediate-release tacrolimus
- Due to reduced clearance and prolonged half-life, patients with severe hepatic impairment (Child-Pugh ≥10) may require a lower starting dosage of ENVARSUS XR
- African American patients, compared with Caucasian patients, may need to be titrated to higher ENVARSUS XR dosages to attain comparable trough concentrations
- Dose adjustments may be necessary when administered concomitantly with CYP3A inducers or inhibitors
Dose once a day2
hidden text
- Administer the first dose in the morning, 12 hours after the patient’s last evening dose of IR-Tac (when converting from twice-daily IR-Tac)
- There is no need to administer any other form of tacrolimus to achieve target trough levels after conversion
- Monitor tacrolimus trough concentrations and titrate ENVARSUS XR dose to achieve the target trough level desired for each patient†
Educate your patients
hidden text
- Be sure they understand exactly how to take ENVARSUS XR and how it is different from the tacrolimus they had been taking
CYP3A=cytochrome P450, family 3, subfamily A; IR-Tac=immediate-release tacrolimus; PK=pharmacokinetic.
†Recommended target whole blood trough concentration range is 4 to 11 ng/mL after the first month.
Use this dose converter to determine the correct dosage of ENVARSUS XR for your patients‡
‡IMPORTANT: Suggested dose has been rounded to an amount that can be achieved with multiples of 1 of the 3 tablet strengths. Actual dose should be determined and adjusted for each individual patient to achieve target trough levels.

See the Convenience Case Study
This case study highlights a patient’s experience with ENVARSUS XR and once-daily dosing.
Download PDFReferences: 1. Nelson J, Alvey N, Bowman L, et al. Consensus recommendations for use of maintenance immunosuppression in solid organ transplantation: endorsed by the American College of Clinical Pharmacy, American Society of Transplantation, and the International Society for Heart and Lung Transplantation. Pharmacotherapy. 2022;42(8):599-633. 2. ENVARSUS XR [package insert]. Cary, NC: Veloxis Pharmaceuticals, Inc.; 4/2024. 3. Food and Drug Administration. Orange Book: approved drug products with therapeutic equivalence evaluations [tacrolimus]. Accessed January 3, 2022. https://www.accessdata.fda.gov/scripts/cder/ob/index.cfm


