Laying the groundwork to achieve target trough early
With ENVARSUS XR, target exposure is achieved quickly—even after a single dose—helping to support your post-transplant monitoring plan.3,4
In an open-label, Phase 2 study of de novo kidney transplant patients, more than half of all patients (53%) achieved target tacrolimus levels (6–11 ng/mL) on Day 2 (pre-dose) after a single starting dose of ENVARSUS XR (0.14 mg/kg/day).3 Therapeutic drug monitoring is recommended to optimize tacrolimus dosing for individual patient needs.3
PATIENTS WITHIN 6 TO 11 ng/mL AFTER THE FIRST DOSE WITH 0.14 mg/kg/DAY
The starting dose of 0.14 mg/kg/day in this study formed the basis of dosing recommendations in de novo kidney transplant patients.3
Demonstrated efficacy with durable outcomes
De novo patients
ENVARSUS XR offers demonstrated efficacy from 3 months to 2 years in de novo kidney transplant patients, providing confidence in results both early post transplant and over time.1,3,5
In head-to-head, noninferiority studies in de novo kidney transplant patients, ENVARSUS XR demonstrated comparable long-term outcomes to PROGRAF®, including BPAR, graft loss, death, and lost to follow-up at 1 and 2 years.1,3
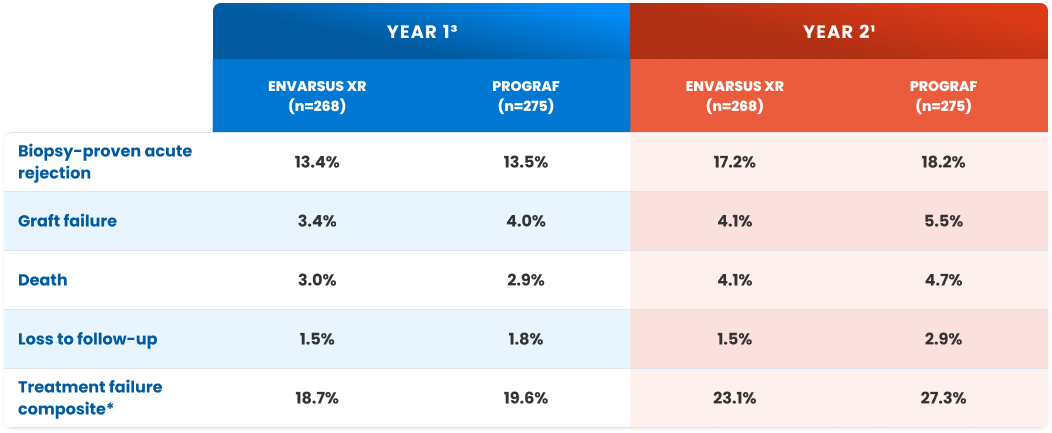
Patient and graft protection at 1 and 2 years1,3
| YEAR 13 | YEAR 21 | |||
|---|---|---|---|---|
| ENVARSUS XR (n=268) | PROGRAF (n=275) | ENVARSUS XR (n=268) | PROGRAF (n=275) | |
| Biopsy-proven acute rejection | 13.4% | 13.5% | 17.2% | 18.2% |
| Graft failure | 3.4% | 4.0% | 4.1% | 5.5% |
| Death | 3.0% | 2.9% | 4.1% | 4.7% |
| Loss to follow-up | 1.5% | 1.8% | 1.5% | 2.9% |
| Treatment failure composite* | 18.7% | 19.6% | 23.1% | 27.3% |
BPAR=biopsy-proven acute rejection.
*Treatment failure was a composite endpoint of BPAR, graft failure, death, and lost to follow-up.1,5
P value not significant for all measures.5
In clinical trials of de novo kidney transplant recipients, the most common adverse events (incidence ≥15%) included: diarrhea, anemia, urinary tract infection, hypertension, tremor, constipation, diabetes mellitus, peripheral edema, hyperkalemia, and headache.
Trial results in patients converting from immediate-release tacrolimus
MELT trial study design2
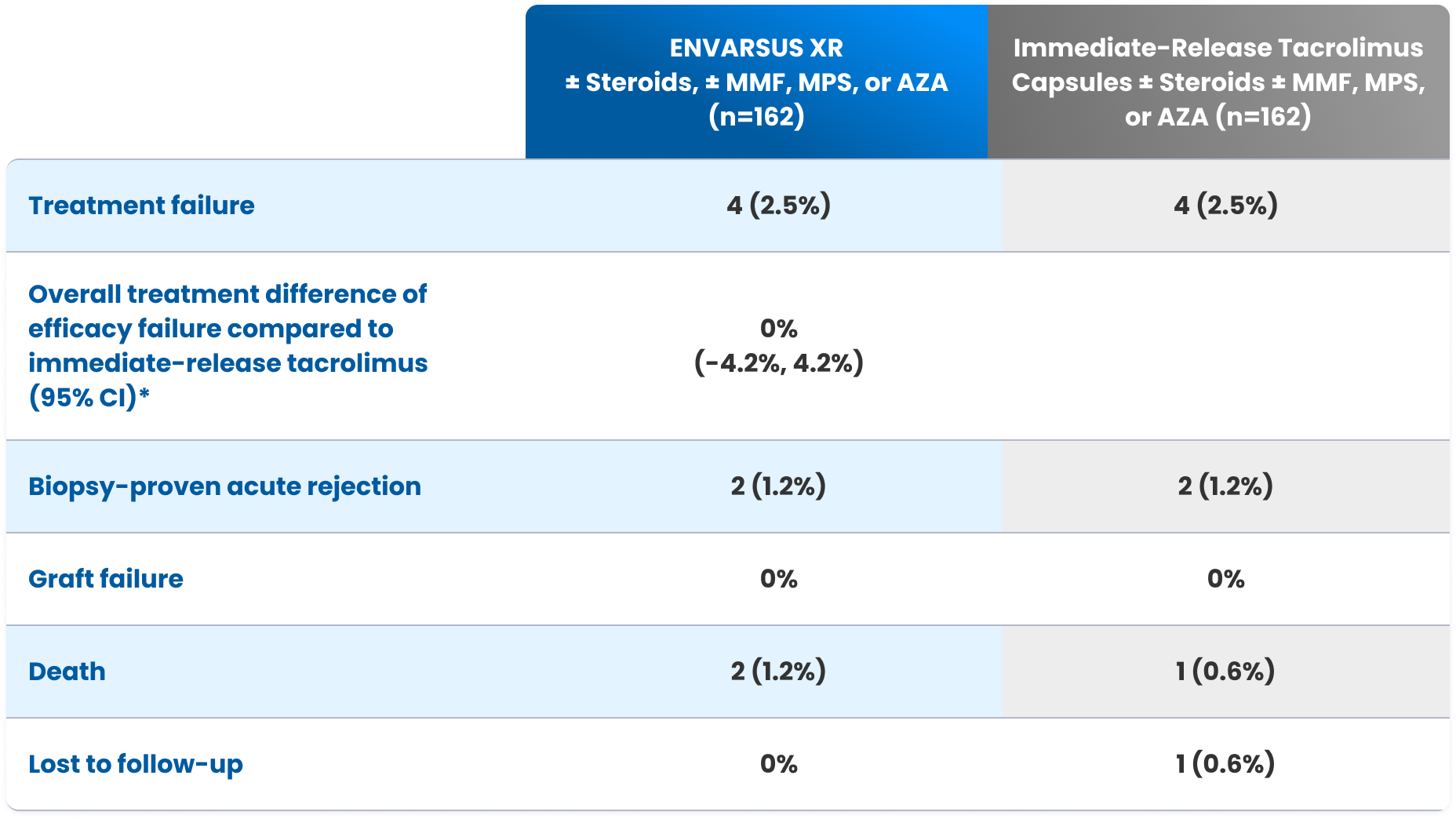
In a controlled, open-label Phase 3 study, 324 adult (aged ≥18 years) renal transplant patients on a stable tacrolimus dose (with trough levels 4-15 ng/mL) were randomized to convert to ENVARSUS XR or remain on maintenance therapy with tacrolimus twice daily (n=162 in each treatment group; modified intent-to-treat population). The primary endpoint was the proportion of patients with efficacy failures (death, graft failure, locally read biopsy-proven acute rejection, or loss to follow-up) within 12 months.
Efficacy failure rates at 12 months3
With its increased bioavailability, a lower dosage of ENVARSUS XR is needed to achieve equivalent therapeutic drug levels and deliver comparable efficacy and safety to immediate-release tacrolimus2
| ENVARSUS XR ± Steroids, ± MMF, MPS, or AZA (n=162) | Immediate-Release Tacrolimus Capsules ± Steroids ± MMF, MPS, or AZA (n=162) | |
|---|---|---|
| Treatment failure | 4 (2.5%) | 4 (2.5%) |
| Overall treatment difference of efficacy failure compared to immediate-release tacrolimus (95% CI)* | 0% (-4.2%, 4.2%) | |
| Biopsy-proven acute rejection | 2 (1.2%) | 2 (1.2%) |
| Graft failure | 0% | 0% |
| Death | 2 (1.2%) | 1 (0.6%) |
| Lost to follow-up | 0% | 1 (0.6%) |
AZA=azathioprine; CI=confidence interval; DGF=delayed graft function; eGFR=estimated glomerular filtration rate; MDRD7=Modification of Diet in Renal Disease 7; MELT=Multicenter Evaluation of LCP-Tacro Trial.; MMF=mycophenolate mofetil; MPS=mycophenolate sodium.
The mean eGFR, using the MDRD7 formula, was 61.5 mL/min/1.73 m2 and 60.0 mL/min/1.73 m2 at baseline (Day 0) and 62.0 mL/min/1.73 m2 and 61.4 mL/min/1.73 m2 at 12 months in the ENVARSUS XR and tacrolimus capsules treatment groups, respectively.
*95% CI was calculated using an exact method that is based on the standardized statistic and inverting a 2-sided test.
In a clinical trial of stable kidney transplant recipients who converted from immediate-release tacrolimus to ENVARSUS XR, the most common adverse reactions (incidence ≥10%) were diarrhea and blood creatinine increased.3
Control from the beginning, control over time
In a Phase 3, randomized, double-blind trial of de novo kidney transplant patients, ENVARSUS XR demonstrated comparable efficacy to PROGRAF.1,5
Freedom From Treatment Failure vs PROGRAF1,6*
HR=hazard ratio.
*Treatment failure was a composite endpoint of BPAR, graft failure, death, and lost to follow-up.1,5
Patients on ENVARSUS XR vs patients on PROGRAF
Experienced a
9% REDUCTION
in treatment failure6†
Experienced a
17% REDUCTION
in treatment failure6†
†Treatment failure was a composite endpoint of BPAR, graft failure, death, and lost to follow-up.1,5

Proven efficacy across patient subgroups
BPAR=biopsy-proven acute rejection; LCPT=LCP-tacrolimus.
*Treatment failure was a composite endpoint of BPAR, graft failure, death, and lost to follow-up.7


See the Control Case Study
This case study highlights the first days of a rapid metabolizer’s post-transplant journey to target tacrolimus levels as quickly as possible.
Download Case StudyA growing foundation of clinical evidence
ENVARSUS XR is built on a robust foundation of evidence—more than 27 trials involving over 1,657 participants.6
Phase 1
19
Trials
516
Healthy Participants
Phase 2
3
Trials
147
Healthy Participants
Phase 3
2
Trials
869
Healthy Participants
Phase 3b
3
Trials
125
Healthy Participants

ENVARSUS XR delivers efficacy with an
established safety profile
Across all safety measures, there were no significant differences between ENVARSUS XR and PROGRAF groups in predefined, potentially clinically significant laboratory measures, opportunistic infections, malignancies, or composite NODAT in both de novo and conversion patients.1,3,5
IR-Tac=immediate-release tacrolimus; NODAT=new-onset diabetes after transplant.


Cultivate Cost Savings for Your Patients
Regardless of your patient’s financial situation, we may have options to help.
Learn moreReferences: 1. Rostaing L, Bunnapradist S, Grinyó JM, et al; Envarsus Study Group. Novel once-daily extended-release tacrolimus versus twice-daily tacrolimus in de novo kidney transplant recipients: two-year results of phase 3, double-blind, randomized trial. Am J Kidney Dis. 2016;67(4):648-659. 2. Bunnapradist S, Ciechanowski K, West-Thielke P, et al; MELT Investigators. Conversion from twice-daily tacrolimus to once-daily extended release tacrolimus (LCPT): the phase III randomized MELT trial. Am J Transplant. 2013;13(3):760-769. 3. ENVARSUS XR [package insert]. Cary, NC: Veloxis Pharmaceuticals, Inc.; 4/2024. 4. Tremblay S, Nigro V, Weinberg J, Woodle ES, Alloway RR. A steady-state head-to-head pharmacokinetic comparison of all FK-506 (tacrolimus) formulations (ASTCOFF): an open-label, prospective, randomized, two-arm, three-period crossover study. Am J Transplant. 2017;17(2):432-442. 5. Budde K, Bunnapradist S, Grinyo JM, et al; Envarsus Study Group. Novel once-daily extended-release tacrolimus (LCPT) versus twice-daily tacrolimus in de novo kidney transplants: one-year results of phase III, double-blind, randomized trial. Am J Transplant. 2014;14(12):2796-2806. 6. Data on file. Veloxis Pharmaceuticals, Inc.;2020. 7. Faravardeh A, Akkina S, Villicana R, et al. Efficacy and safety of once-daily LCP-tacrolimus versus twice-daily immediate-release tacrolimus in adult Hispanic stable kidney transplant recipients: sub-group analysis from a phase 3 trial. Ann Transplant. 2021;16:26:e929535.


